Top Three
FDA Delays Review on Vaccine for Under 5s: Pfizer and BioNTech today announced they were delaying the application process for an emergency use authorization (EUA) for their vaccine for children ages 6 months to 4 years old, and gathering more information on two and three doses of the vaccine.
"The trial in children 6 months through 4 years of age is ongoing and data on the first two 3 µg doses in this age group are being shared with the FDA on an ongoing basis. Cases continue to accumulate according to the study protocol and more data are being generated because rates of infection and illness remain high in children of this age, especially due to the recent Omicron surge."
"Given that the study is advancing at a rapid pace, the companies will wait for the three-dose data as Pfizer and BioNTech continue to believe it may provide a higher level of protection in this age group. This is also supported by recent observations of three dose booster data in several other age groups that seems to meaningfully augment neutralizing antibody levels and real world vaccine protection for omicron compared to the two-dose regimen."
"The companies expect to have three-dose protection data available in early April."
"The independent Data Monitoring Committee (DMC) for the study supports the continuation of the trial according to the protocol and believe that the data collected to date indicate the vaccine is well tolerated and support a potential three-dose regimen."
The FDA: "As part of its rolling submission, the company recently notified the agency of additional findings from its ongoing clinical trial...Based on the agency's preliminary assessment, and to allow more time to evaluate additional data, we believe additional information regarding the ongoing evaluation of a third dose should be considered as part of our decision-making for potential authorization.”
NBC News reports, "Two people familiar with the FDA’s plans said there had already been a lot of pushback on the agency from outside experts who had concerns that Pfizer’s data wasn’t sufficient. The experts felt, one of the people said, that their concerns were “falling on deaf ears” within the agency."
"In a briefing for journalists on the decision, the FDA's Peter Marks appeared to hint that it was because the data provided by the companies weren’t strong enough to warrant early authorization."
"Norman Baylor, who formerly held Marks’ position at the FDA, was one of those who felt it would be ill-advised to start using the vaccine in children under 5 before the trial had concluded."
"Some sources, who spoke on condition of anonymity, said data from Pfizer to the FDA had come in late, and FDA staffers may have needed more time to vet them. It’s also possible new issues emerged with those data."
Emily Oster speaks for all of us with "WTF is Going on?"
"I don’t know, and I’m not sure anyone else outside the room really does either. There are really two options. One is that something emerged in the data review that was concerning. I think this is very unlikely, in part because Pfizer expressed confidence in approval after sending in the submission."
"Instead, what I think is likely is that -- as we knew in December -- the two-dose series did not provide a very strong immune response in the 2 to 5 year olds. The rumblings about efficacy against symptomatic illness suggested a 50 to 60% reduction in illness risk, and that this was lower with Omicron than with Delta. This would be a reasonable but not amazing efficacy level."
"As they contemplated this, they likely faced significant push back, internally and externally. My best -- totally speculative -- guess is that they were concerned that the EUA would be rejected by their panel, and this would be embarrassing and damaging. So they pulled the plug."
My quick take: A number of folks emailed asking if this was an issue around the safety of the vaccine or its efficacy. It looks to be more of an efficacy issue made more complicated by the Omicron variant. If it was a safety issue, Pfizer's statement wouldn't have included a note from DMC saying the data suggest "the vaccine is well tolerated." And we wouldn’t see them give a new date for review (April). It also looks like the FDA wasn't confident they had the votes among the advisory committee members - so rather than tee up a bad vote, they decided to wait for the full set of data of three doses.
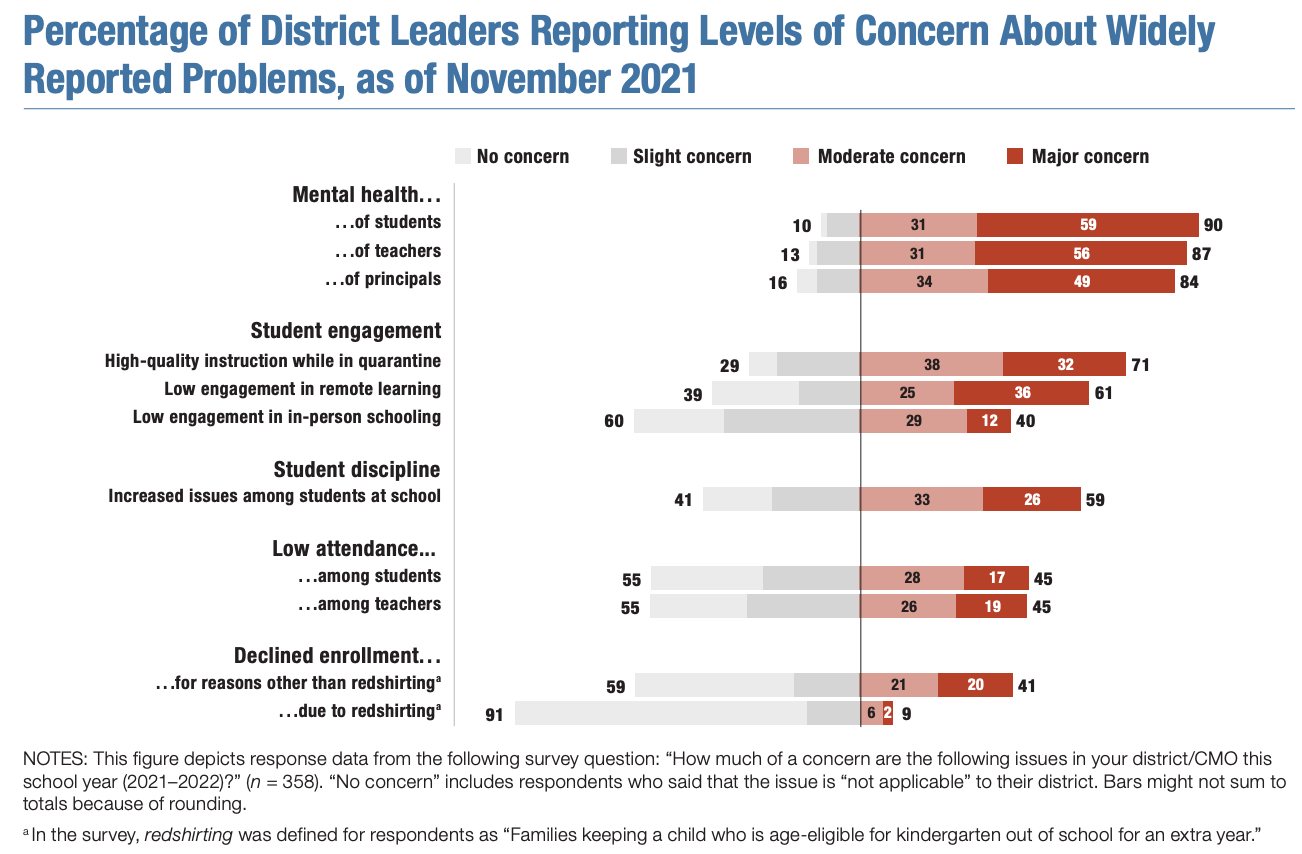
District Leaders' Concerns About Mental Health and Political Polarization in Schools: Findings from the Fourth American School District Panel Survey
90% of district leaders expressed either “moderate” or “major” concern about students’ mental health this school year. And 87 and 84% of district leaders, respectively, reported the same levels of concern about teachers and principals.
71% of district leaders reported “moderate” or “major” concern about providing high-quality instruction to quarantined students.
6% of students, on average, were absent on a typical school day at the time they took the survey because of quarantines related to COVID-19.
COVID Growing Less Lethal: FT visualizes most recent UK data.
Federal
HHS: Announces Winners of National Challenge to Increase Pediatric Vaccinations and Well-Child Visits.
COVID-19 Research
New Antibody Drug Approved: FDA authorizes new Eli Lilly and Company antibody drug that stands up against Omicron. FDA Announcement / Eli Lilly Press Release.
Protection Drops Four Months After Third Dose: CDC's Morbidity and Mortality Weekly Report features a study showing that protection against Omicron hospitalizations and emergency department visits drops significantly by 4 months after a third booster dose but is overall still high.
"The authors found vaccine effectiveness (VE) during the Omicron-predominant period was 87% against emergency department or urgent care (ED/UC) visits and 91% against hospitalization during the 2 months following a booster dose of vaccine."
"VE dropped to 66% and 78% against COVID-19–associated ED/UC visits and hospitalizations, respectively, at 4 months after a booster dose."
Analysis of Vaccination Rates and New COVID-19 Infections by US County, July-August 2021: New study which found the counties with the highest rates of vaccination against Covid-19 had the lowest case totals.The epidemic grew the most in southern states while most counties with low vaccination rates were rural.
The US Still Isn't Getting Covid-19 Data Right: Via CNN.
"That was the impetus, and that's why we're continuing to do it, because we haven't seen a replicated resource at a global level or a domestic level that has brought in the same kind of fidelity to that governance model," said Beth Blauer, executive director of the Centers for Civic Impact at Johns Hopkins University and data lead for the Coronavirus Resource Center."
"Inconsistencies remain. For example, some states count new Covid-19 cases by person, while others report new cases based on the total number of positive tests, regardless how many times one person may have tested positive.
"Some states report only PCR tests, while others include positive antigen tests, too."
"The surveillance worksheet that the CDC provides for health departments to report Covid-19 cases spans six pages and has more than 300 fields for data entry."
Vaccine Hesitancy: Via Axios
"CDC data shows fewer than 100,000 people are getting their first COVID vaccine dose every day, the lowest daily average since the vaccines became available in late 2020."
State
DC: Early results from DC' Public Schools required weekly covid tests for pre-K students: approximately 3,910 students in both prekindergarten levels submitted results — with 43 testing positive.
Massachusetts:
New Jersey: Students who lost special ed services due to COVID can get more help.
"Most parents have until around March 18 — the second anniversary of the day most New Jersey schools switched to remote learning at the start of the pandemic — to file a claim for a hearing in the state Office of Administrative Law covering the full time schools were closed."
North Carolina: Health officials on eased guidance for K-12 schools that had directed students and staff to often stay home for five days if they were in close contact with someone who tests positive for COVID-19.
"Nearly all 115 school boards agreed to mask mandates to start the current school year, but boards that have approved mask-optional policies had grown to nearly 30 by late last week."
Oregon: Schools to make own mask requirements starting March 31.
Resources
A Top Researcher Says it's Time to Rethink Our Entire Approach to Preschool: Via NPR
Schools Lift Mask Requirements, Change Covid-19 Rules as Omicron Recedes: Via WSJ.
Exits by Black and Hispanic Teachers Pose a New Threat to Covid-Era Education: Via KHN
Will Schools Require Covid-19 Vaccines for Students?: Asks Vox.
"Children are less likely to become severely ill from Covid-19, perhaps decreasing the urgency of vaccination for some families. What’s more, parents are often more anxious and cautious about their children’s health than their own, said Richard Meckel, an emeritus professor of American studies at Brown, who has studied the history of childhood and health policy. Parents who were willing to get vaccinated themselves may be more concerned about side effects when it comes to their children."
"A number of districts, like those in Oakland and San Diego, announced vaccine mandates for students and then pushed back their deadlines, said Bree Dusseault, principal at the Center on Reinventing Public Education (CRPE), which has been tracking Covid-19 policies at 100 large districts nationwide. In many cases, the reason is simply low vaccination rates. In Oakland, for example, so many children remained unvaccinated that it would have been impossible to accommodate them at the district’s virtual school."
"Meanwhile, 17 states have taken the opposite approach, banning Covid-19 vaccine mandates in schools. In these states, which include Texas, Georgia, and Florida, districts couldn’t require the vaccine even if they wanted to."
Nelson Mandela Released From Prison: On this day 27 years ago.
Her Dad Died. So Her Favorite NFL Star Took Her to the Father-Daughter Dance: Via the Washington Post.
"Soape sent an Instagram message a few days before Christmas to Anthony Harris, a Philadelphia Eagles player who is originally from Richmond."
“Hey, this is kind of a crazy big ask, and please feel free to say no, but there’s this dance at the end of January,” Soape wrote to the NFL player, explaining her daughter’s predicament. “Is there any way you would consider doing this?”
“If we don’t make it to the playoffs, I would be open to that,” he wrote back to Soape."
“I think I might have been a little more nervous than she was,” Harris confessed with a laugh."
“I just wanted to try to help her cope through that experience without her father being there,” he said. “I had people in my life, sometimes complete strangers, that were very supportive of me, so I wanted to do that for her.”
We Are Not a Nation of Soloists, but a Chorus of Shared Values: Via Steve Hartman, "Every year 600 of the best high school singers from Kentucky gather to practice and perform at Louisville’s convention center – but it’s the tradition that started in a hotel that leaves those who get a chance to listen in awe."